A flexible and elastic battery for foldable smartphones
To meet the needs of thinner and more flexible devices, researchers at a Zurich university have succeeded in creating a battery that can bend and lie down. Better yet, it would be much safer for the environment than the usual lithium-ion batteries.
A team of researchers from the Swiss Federal Institute of Technology Zurich (ETHZ) has developed a new battery. Current accumulators, generally of the lithium-ion type, are rigid and heavy. In a context where the industry is developing more and more flexible electronic devices, such as foldable smartphones or clothing, scientists have succeeded in replacing the elements that usually constitute a battery with elastic materials.
The researchers presented their battery, currently in prototype form, in a video. Unlike any existing battery, it can be bent and even stretched, which finally promises a power source compatible with new formats of devices or flexible electronic circuits
A water-based electrolyte
The battery differs thanks to a new electrolyte, the conductive substance that connects the anode and the cathode of the battery, most often in the liquid state. It is a water-based gel, with a highly concentrated lithium salt that not only improves the conductivity during charging or discharging, but also prevents the electrochemical decomposition of water. This new electrolyte also promises more environmentally friendly batteries, those used usually being very toxic and highly flammable.
The researchers combined this gel with other flexible materials to create their battery, combined in many layers like a sandwich. The current collectors for the anode and the cathode consist of a flexible composite polymer which contains conductive carbon and which also constitutes the shell. They covered the inner surface of the polymer with silver flakes the size of a micron overlapping in tile-like manner on a roof. This allows them to stay in contact despite the deformations of the polymer and to ensure the conductivity inside the battery.
What is special about this new battery is its electrolyte, the part of the battery through which lithium ions pass when charged or discharged © ETH Zurich
A safe battery in the event of a leak
The presence of carbon will allow the current to pass through the polymer in extreme cases where the contact between the silver flakes would be broken, even if the conductivity will be less good. The anode and the cathode consist of powders which have been sprayed on the silver layer.
In the case of the anode, it is vanadium oxide, while the cathode uses an oxide of lithium and manganese. The two polymer layers, one containing one side with the anode, the other with the cathode, are then separated by a frame layer with a hole in the middle filled with the electrolyte gel.
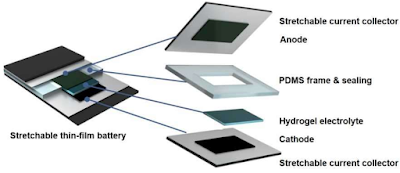 |
The battery is composed as a sandwich from a variety of different flexible materials © ETH Zurich |
The different layers of the current prototype are fixed together thanks to an adhesive that demonstrates the elasticity of the battery. However, the researchers will eventually have to replace it, as Prof. Markus Niederberger, professor at ETH Zurich explains: "If we want to market the battery, we will have to find another process that will keep it closed for longer."
The team is already imagining many applications, such as roll-up screens for computers, foldable smartphones or even the ability to sew the battery directly into connected clothes. In addition, if the battery is damaged, leaking liquid will be safe.















No comments:
Post a Comment